Difference between revisions of "Anomalous absorption"
From Online Dictionary of Crystallography
BrianMcMahon (talk | contribs) m |
BrianMcMahon (talk | contribs) m (Tidied translations.) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | < | + | <font color="blue">Absorption anomale</font> (''Fr''). <font color="red">Anomale Absorption</font> (''Ge''). <font color="black">Assorbimento anomalo</font> (''It''). <font color="green">Absorción anómala</font> (''Sp''). |
== Definition == | == Definition == | ||
| Line 19: | Line 19: | ||
== See also == | == See also == | ||
| − | + | *[[Borrmann effect]] | |
| − | + | *Chapter 5.1 of ''International Tables for Crystallography, Volume B'' for X-rays | |
| − | + | *Chapter 5.2 of ''International Tables for Crystallography, Volume B'' for electrons | |
| − | + | *Chapter 5.3 of ''International Tables for Crystallography, Volume B'' for neutrons | |
| − | |||
| − | |||
[[Category:X-rays]] | [[Category:X-rays]] | ||
Latest revision as of 17:54, 8 November 2017
Absorption anomale (Fr). Anomale Absorption (Ge). Assorbimento anomalo (It). Absorción anómala (Sp).
Definition
Anomalous absorption takes place when radiation is dynamically diffracted by a perfect or nearly perfect crystal. The optical field in the crystal is then made up of several components, called wavefields, two in the two-beam case (neglecting polarization in the X-ray case). One of them is absorbed more than normal and the other one less than normal. In the transmission, or Laue, geometry, both wavefields propagate inside the crystal; one then speaks of anomalous transmission for the less absorbed wavefield (Borrmann effect). In the reflection, or Bragg, geometry, one wavefield only propagates in the crystal, the more absorbed one for angles of incidence corresponding to one side of the total reflection rocking curve and the less absorbed one for the other side. This results in an asymmetry of the rocking curve that is calculated using dynamical theory.
Physical interpretation
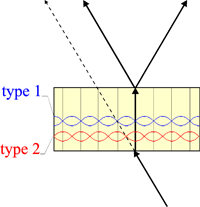
Borrmann (1950) interpreted anomalous absorption by means of the standing waves that are formed by the interference of the incident and reflected waves (Laue, 1937, 1941). The nodes and antinodes of these standing waves have the periodicity of the lattice planes. In the transmission geometry (see figure), the nodes lie on the lattice planes for type 1 wavefields, which undergo therefore a quite small absorption, while it is the antinodes that lie on the lattice planes for type 2, which undergo a larger absorption.
In the reflection geometry, the nodes and antinodes shift by half a lattice distance as the crystal is rocked through the rocking curve. On the smaller angle side of the rocking curve it is type 1 wavefields that are excited, the nodes lie on the lattice planes and the absorption is small. On the higher angle side of the rocking curve, it is type 2 wavefields that are excited, the antinodes lie on the lattice planes and the absorption is large. This effect is used in the standing-wave technique for observing adsorbed atoms at the surface of crystals.
History
Anomalous absorption was discovered by Borrmann on quartz [Borrmann G. (1941). Physik Z., 42, 157-162. Über Extinktionsdiagramme der Röntgenstrahlen von Quarz] and calcite crystals [Borrmann G. (1950). Z. Phys., 127, 297-323. Die Absorption von Röntgenstrahlen in Fall der Interferenz] and first interpreted by Laue [Laue, M. von (1949). Acta Cryst., 2, 106-113. Die Absorption der Röntgenstrahlen in Kristallen im Interferenzfall].
See also
- Borrmann effect
- Chapter 5.1 of International Tables for Crystallography, Volume B for X-rays
- Chapter 5.2 of International Tables for Crystallography, Volume B for electrons
- Chapter 5.3 of International Tables for Crystallography, Volume B for neutrons