Reciprocal lattice
From Online Dictionary of Crystallography
Réseau réciproque (Fr). Reziprokes Gitter (Ge). Reticolo reciproco (It). 逆格子 (Ja). Red recíproca (Sp).
Contents
Definition
The reciprocal lattice is constituted of the set of all possible linear combinations of the basis vectors a*, b*, c* of the reciprocal space. A point (node), H, of the reciprocal lattice is defined by its position vector:
OH = r*hkl = h a* + k b* + l c*.
If H is the nth node on the row OH, one has:
OH = n OH1 = n (h1 a* + k1 b* + l1 c*),
where H1 is the first node on the row OH and h1, k1, l1 are relatively prime.
The generalization of the reciprocal lattice in a four-dimensional space for incommensurate structures is described in Chapter 9.8 of International Tables for Crystallography, Volume C.
Geometrical applications
Each vector OH = r*hkl = h a* + k b* + l c* of the reciprocal lattice is associated with a family of direct lattice planes. It is normal to the planes of the family, and the lattice spacing of the family is d = 1/OH1 = n/OH if H is the nth node on the reciprocal lattice row OH. One usually sets dhkl = d/n = 1/OH. If OP = x a + y b + z c is the position vector of a point of a lattice plane, the equation of the plane is given by OH1 ⋅ OP = K where K is a constant integer. Using the properties of the scalar product of a reciprocal space vector and a direct space vector, this equation is OH1 ⋅ OP = h1x + k1y + l1z = K. The Miller indices of the family are h1, k1, l1. The subscripts of the Miller indices will be dropped hereafter.
The Miller indices of the family of lattice planes parallel to two direct space vectors, r1 = u1 a + v1 b + w1 c and r2 = u2 a + v2 b + w2 c are proportional to the coordinates in reciprocal space, h, k, l, of the vector product of these two vectors:
h/(v1 w2 - v2 w1) = k/(w1 u2 - w2 u1) = l/(u1 v2 - u2 v1).
The coordinates u, v, w in direct space of the zone axis intersection of two families of lattice planes of Miller indices h1, k1, l1 and h2, k2, l2, respectively, are proportional to the coordinates of the vector product of the reciprocal lattice vectors associated with these two families:
u/(k1 l2 - k2 l1) = v/(l1 h2 - l2 h1) = w/(h1 k2 - h2 k1).
Centred lattices
| Direct lattice | Reciprocal lattice | ||||
|---|---|---|---|---|---|
| Bravais letter | Centring vectors | Unit-cell volume Vc | Bravais letter | Multiple unit cell | Unit cell volume V*c |
| P | 0 | V | P | a*c, b*c, c*c | V* |
| A | ½bc+½cc | 2V | A | a*c, 2b*c, 2c*c | ½V* |
| B | ½cc+½ac | 2V | B | 2a*c, b*c, 2c*c | ½V* |
| C | ½ac+½bc | 2V | C | 2a*c, 2b*c, c*c | ½V* |
| I | ½ac+ ½bc +½cc | 2V | F | 2a*c, 2b*c, 2c*c | ½V* |
| F | ½ac+ ½bc | 4V | I | 2a*c, 2b*c, 2c*c | ¼V* |
| ½bc+ ½cc | |||||
| ½cc+ ½ac | |||||
| R | 0 | V | R | a*c, b*c, c*c | V* |
| (rhombohedral axes) | |||||
| R | ⅔a*c + ⅓b*c + ⅓c*c | 3V | R | 3a*c, 3b*c, 3c*c | ⅓V* |
| (hexagonal axes) | ⅓a*c + ⅔b*c + ⅔c*c | ||||
where ac, bc, cc are the basis vectors of the conventional multiple cell and a*c, b*c, c*c the corresponding reciprocal lattice vectors.
An elementary proof that the reciprocal lattice of a face-centred lattice F is a body-centred lattice I and, reciprocally, is given in The Reciprocal Lattice (Teaching Pamphlet No. 4 of the International Union of Crystallography).
Diffraction condition in reciprocal space
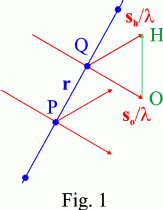
The condition that the waves outgoing from two point scatterers separated by a lattice vector r = u a + v b + w c (u, v, w integers) be in phase is that the scalar product (sh/λ - so/λ) ⋅ r, where sh and so are unit vectors in the scattered and incident directions, respectively, be an integer, n. This condition is satisfied whatever r if the diffraction vector (OH = sh/λ - so/λ) is of the form:
(sh/λ - so/λ) = h a* + k b* + l c*,
where h, k, l are integers, namely the diffraction vector OH is a vector of the reciprocal lattice (Fig. 1).
A node of the reciprocal lattice is therefore associated with each Bragg reflection on the lattice planes of Miller indices (h/K, k/K, l/K). It is called the hkl reflection.
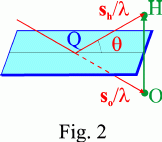
The relation sh/λ - so/λ = OH generalizes the Laue equations. It is equivalent to Bragg's law, as can be seen in Fig. 2.
Consider the lattice plane passing through lattice point Q and perpendicular to reciprocal-lattice vector OH and let θ be the angle between the incident, so, or the reflected, sh, directions and the lattice plane. It can be seen from the figure that
OH/2 = sin θ/λ,
and, since OH = n/d (d lattice spacing of the family of lattice planes associated with OH) and dhkl = d/n:
2 d sin θ = n λ, or 2 dhkl sin θ = λ,
which is Bragg's law. n is the order of the reflection.
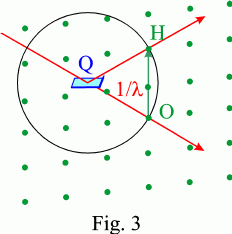
Another way to express the diffraction condition in reciprocal space is to consider a sphere centred at a node Q of the direct lattice, of radius 1/λ and passing through the origin O of the reciprocal lattice (Fig. 3). If it passes through another node, H, of the reciprocal lattice, Bragg's law is satisfied for the family of direct lattice planes associated with that node and of lattice spacing dhkl = n/OH if H is the nth node on the row OH (n = 2 in the example of Fig. 3). This sphere is called the Ewald sphere.
History
The notion of reciprocal vectors was introduced in vector analysis by J. W. Gibbs [(1881). Elements of Vector Analysis, arranged for the Use of Students in Physics. Yale University, New Haven; reprinted: Gibbs, J. W. and Wilson, E. B. (1902). Vector analysis, New York; 1960, Dover Publications]. The concept of reciprocal lattice was adapted by P. P. Ewald to interpret the diffraction pattern of an orthorhombic crystal (1913) in his famous paper where he introduced the sphere of diffraction. It was extended to lattices of any type of symmetry by M. von Laue (1914) and Ewald (1921). The first approach to that concept is that of the system of polar axes, introduced by Bravais in 1850, which associates the direction of its normal with a family of lattice planes.
See also
- Direct lattice
- Polar lattice
- Reciprocal space
- The Reciprocal Lattice (Teaching Pamphlet No. 4 of the International Union of Crystallography)
- Chapter 1.3.2.5 of International Tables for Crystallography, Volume A, 6th edition
- Chapter 1.1 of International Tables for Crystallography, Volume B
- Chapter 1.1 of International Tables for Crystallography, Volume C
- Chapter 1.1.2 of International Tables for Crystallography, Volume D